Sie befinden sich hier
Inhalt
Projects
A) Influence of Measles Virus infection of monocyte-derived dendritic cells on interferon regulatory factors.
Measles virus (MeV) is a negative stranded RNA virus belonging to the family of Paramyxoviridae. It is a highly contagious human pathogen that is transmitted by aerosols and characterised by cough, coryza, fever, often conjunctivitis and later on by a characteristic exanthema. Arising of the exanthema also marks the beginning of the hosts immune response. Although MeV induces a strong immune reaction, resulting in a life-long immunity against reinfection, a measles-associated immune suppression can be observed weeks to months after an acute infection. This immune suppression leads to a higher susceptibility to secondary bacterial infection and increases the risk of complications like diarrhoea, pneumonia or otitis media.
Although an effective life-attenuated vaccine against infection with MeV exists, measles still belong to the leading causes of death among children under five years.
WHO still counted about 134.000 deaths due to measles infection in 2015 worldwide.
Tissue resident dendritic cells and alveolar macrophages are thought to be the first targets of MeV. During infection SLAM-positive lymphocytes and dendritic cells are infected, whereas epithelial cells are thought to be infected late in infection.
To detect pathogen-associated-molecular-patterns (PAMPs) expressed on invading pathogens dendritic cells use intracellular sensors for virus-specific RNAs. As a result the production of type-I-interferons is induced and the transcription of various antiviral genes is encouraged.
Our research aims to identify strategies of MeV to circumvent the host immune system and to induce the temporary immune suppression. Especially, we are interested in the interaction of measles virus with cells of the immune system, in particular dendritic cells.
Here, we investigate the ability of MeV vaccine strains in comparison to different MeV wild-type strains to modify the expression and function of different interferon inducing genes.
B) Silencing of chronically activated cells in neurodegenerative diseases by an anti-inflammatory gene-therapeutic approach
Our research interests are focused on the characterisation and regulation of inflammatory processes in the central nervous system (CNS). Invasion of the CNS by pathogens can results in a rapid local activation of immune cells. Hence, they produce numerous pro-inflammatory substances, including cytokines, chemokines, reactive oxygen species (ROS) and nitric oxide (NO). To a certain extent these reactions have protective functions, but they have to be controlled and limited to prevent detrimental effects on neurons.
Inflammatory events in the CNS are regulated by receptor-ligand pairs, so called “neuroimmune regulators” (NiRegs). While the expression of the receptors is restricted to specialized cells (e.g. neurons) the ligand is expressed on a variety of different cells, like cells of myeloid origin. This leads to a very well adjusted signaling compared to soluble substances.
In neurological disorders a dysfunction or down regulated expression of NiRegs is often observed. Thisresults in a poorly controlled reaction of the local immune system and a constant production of pro-inflammatory substances. Consequently, neurons will be impaired. Interestingly, viruses of the pox- and herpesviridae are known to encode for homologues of several cytokines and also soluble homologues of NiRegs. They therefore gain advantages in establishing a productive infection in their host by suppressing the host’s immune response.
In our research projects we aim to adopt strategies of viruses to slow down immune responses after experimentally induced activation of different cells of the immune system. We are using a retroviral vector system to produce virus like particles (VLPs) that contain an (neuro-) immune regulatory transgene. These VLPs are used to deliver the transgene into experimentally activated immune cells of the central nervous system. The potential of soluble (neuro-) immune regulatory proteins to suppress or down regulate the inflammatory response of activated immune cells is our main interest.
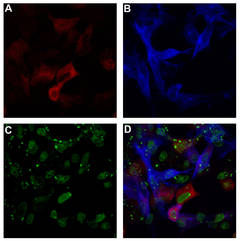
FIV infected cells co-expressing a neuroimmune regulator (NiReg).
A: Intracellular staining of the FIV p24 protein. B: intracellular staining of co-transfected NiReg. C: Staining of the nuclei. D: Overlay of A-C.
Publications:
Seyer, R.; Hrincius, E.R.; Ritzel, D.; Abt, M. 1); Mellmann, A.; Marjuki, H.; Kühn, J.; Wolff, T.; Ludwig, S.; Ehrhardt, C.
Synergistic adaptive mutations in the HA and PA lead to increased virulence of pandemic
2009 H1N1 influenza A virus in mice.
J Infect Dis. 2012 Jan 15;205(2):262-71. Epub 2011 Nov 18.
Abt, M. 1); Laue, M.; Wolff, T.
Improvement of H5N1 influenza vaccine viruses: influence of internal gene segments of
avian and human origin on production and hemagglutinin content.
Vaccine. 2011 Jul 18;29(32):5153-62.
Abt, M. 1); Gassert, E.; Schneider-Schaulies, S.
Measles virus modulates chemokine release and chemotactic responses of dendritic cells.
J Gen Virol. 2009 Apr;90(Pt 4):909-14. Epub 2009 Mar 4.
Wolff, T.; Zielecki, F.; Abt, M.; Voss, D.; Semmler, I.; Matthaei, M.
Sabotage of antiviral signaling and effectors by influenza viruses.
Biol Chem. Oct; 389(10):1299-305.
De Witte, L.*; Abt, M.* 1); Schneider-Schaulies, S.; van Kooyk, Y.; Geijtenbeek, T.
Measles Virus targets DC-SIGN to enhance DC infection.
J Virol. 2006 Apr;80(7):3477-86.
*Autoren haben gleichberechtigt an der Arbeit mitgewirkt
Abt, M. 1); Müller, N.; Schneider-Schaulies, S. (2005).
Dendritic cells in measles pathogenesis. In: Handbook of Dendritic Cells, disease and
therapies, 2 Vol. Steinkasserer, A.; Lutz, M. (eds.) Wiley VCH, Deutschland
Klagge, I.M.; Abt, M1).; Fries, B.; Schneider-Schaulies, S. (2004).
Impact of measles virus on dendritic cell infection on T cell polarisation in vitro.
J Gen Virol. 2004 Nov;85(Pt 11):3239-47.
1) married: Kopčák, M.
Kontextspalte
Leitung

Univ. Prof. Dr. med. Thomas Miethke
Direktor des Instituts
Sekretariat
Telefon 0621/383-2224
Telefax 0621/383-3816
sekretariat-immh@umm.de
Universitätsmedizin Mannheim
Theodor-Kutzer-Ufer 1-3
68167 Mannheim


