Sie befinden sich hier
Inhalt
Interfering with TGF-β2: the next approach of targeted CLD therapy?
In CLD, tissue remodeling and wound healing are disturbed based on a complex modulation of cell-cell communication and intracellular signaling processes. The resulting fibrogenesis called process is the onset of liver scarring, cirrhosis and the development of hepatic failure. Currently, liver transplantation is the only therapeutical intervention for terminal liver disease. The demand for new therapies increases due to donor organ limitations and enormous economic costs.
The TGF-β pathway has emerged as a putative anti-cancer therapeutic target, but TGF-β is also implicated in many physiological and pathophysiological processes related to several human diseases other than cancer including fibrosis. TGF-β acts as a cytostatic factor on epithelial cells, e.g. on proliferating hepatocytes during liver regeneration. However, it may become an oncogenic factor, facilitating proliferation, angiogenesis and invasion. Compounds able to target the TGF-β pathway have been developed by many basic research labs and several pharmaceutical companies, however, still require validation and results were not robust enough for efficient clinical translation.
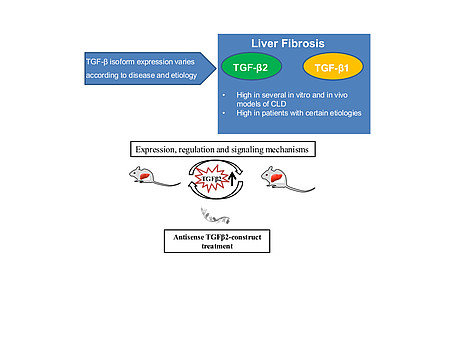
Different strategies include antisense oligonucleotides or small inhibitor molecules. Currently, LY2157299, an ALK5 inhibitor, is used to interfere with these activities in HCC patients. In mammals, TGF-β exists in three isoforms, TGF-β1, TGF-β2 and TGF-β3. Understanding cell type and disease stage associated molecular mechanisms of its action is essential to develop therapeutic strategies.
My research follows on delineating variance in TGF-β isoform function in chronic liver diseases with focus on specific inhibition of TGF-β2 and/or TGF-β1 with antisense oligonucleotides (AONs), specifically designed short DNA or RNA stretches, which downregulate gene expression. TGF-β1 is highly expressed in most fibrotic tissues, including liver. Expression and function of TGF-β2 has not been investigated thoroughly in CLD progression and HCC, and is subject matter of my project.
Further, we aim at developing therapeutic strategies to selectively target TGF-β1 and TGF-β2 in human patients with liver disease. I will therefore investigate function and targeting of TGF-β2, as compared to TGF-β1 in HCC cell lines, hepatocytes and hepatic stellate cells (HSC; the latter being the major focus of my work), as well as in animal models of CLD (CCl4, BDL, Mdr2-/-), especially using the aforementioned AONs. I will identify their contribution to HSC activation and liver fibrogenesis. The antisense approach against TGF-β will be evaluated for efficacy and treatment modalities in preclinical models and, hopefully, then further translated in a clinical trial.
Dropmann A. et al. . TGF-β1 and TGF-β2 abundance in liver diseases of mice and men. Oncotarget 2016 DOI: 10.18632/oncotarget.6967
Emancipation of hepatic TGF-β2:Anti-fibrotic and anti-inflammatory consequences of TGF-β2 silencing in biliary liver disease, Manuscript in preparation
Kontextspalte